Osmosis Definition And Explanation in Plants
What is Osmosis?
Osmosis is the net movement of water molecules from a region of higher water potential (lower solute concentration) to a region of lower water potential (higher solute concentration) across a partially permeable membrane.
Key Definitions
Water Potential (Ψ)
- Measure of the potential energy of water
- Determines direction of water movement
- Pure water has water potential of 0 (highest)
- Adding solutes decreases water potential (becomes negative)
- Water always moves from less negative to more negative water potential
Partially Permeable Membrane
- Allows water molecules to pass through
- Prevents larger solute molecules from passing
- Examples: Cell membrane, visking tubing, dialysis membrane
Solute
- Substance dissolved in water
- Examples: Salt, sugar, ions
- Increases osmotic pressure
Solvent
- Liquid that dissolves solutes
- In biological systems, usually water
Osmosis in Plant Cells
The Cell Membrane as a Partially Permeable Membrane
Plant cells have a cell membrane (plasma membrane) that acts as a partially permeable barrier between the cytoplasm and the external environment. This membrane:
- Allows free passage of water molecules
- Controls movement of dissolved substances
- Maintains cellular homeostasis
Water Potential in Plant Cells
Plant cells contain:
- Dissolved salts and minerals
- Sugars and organic compounds
- Proteins and other macromolecules
These solutes lower the water potential inside the cell, making it negative compared to pure water.
Typical water potential values:
- Pure water: 0 kPa
- Soil water: -10 to -100 kPa
- Plant cell cytoplasm: -100 to -1000 kPa
- Air (dry): -100,000+ kPa
The Role of the Cell Wall
Unlike animal cells, plant cells have a rigid cell wall outside the cell membrane:
- Made of cellulose
- Fully permeable to water and solutes
- Provides structural support
- Prevents cell bursting when water enters
Effects of Osmosis on Plant Cells
Three Possible Scenarios
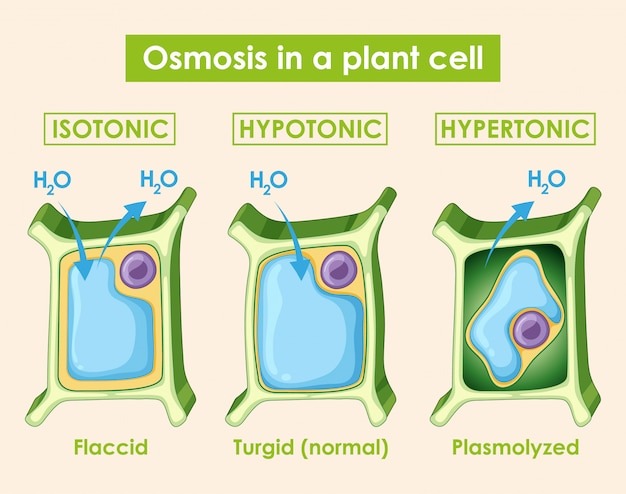
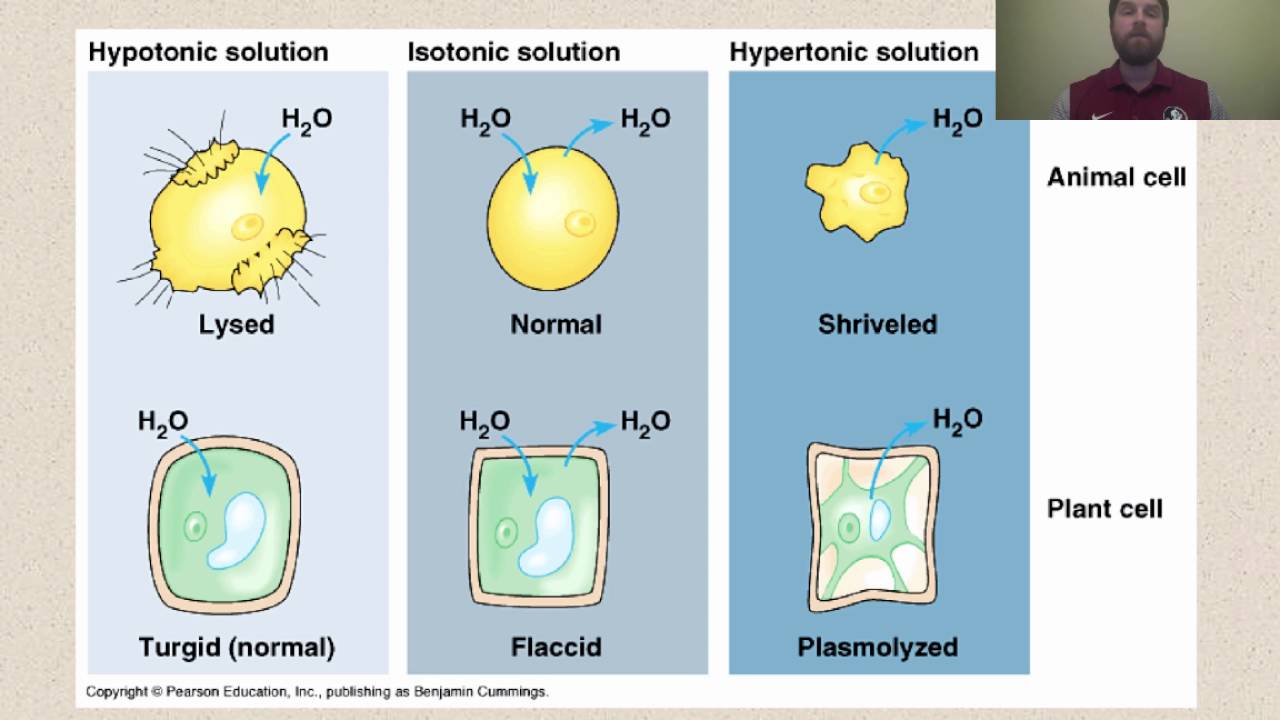
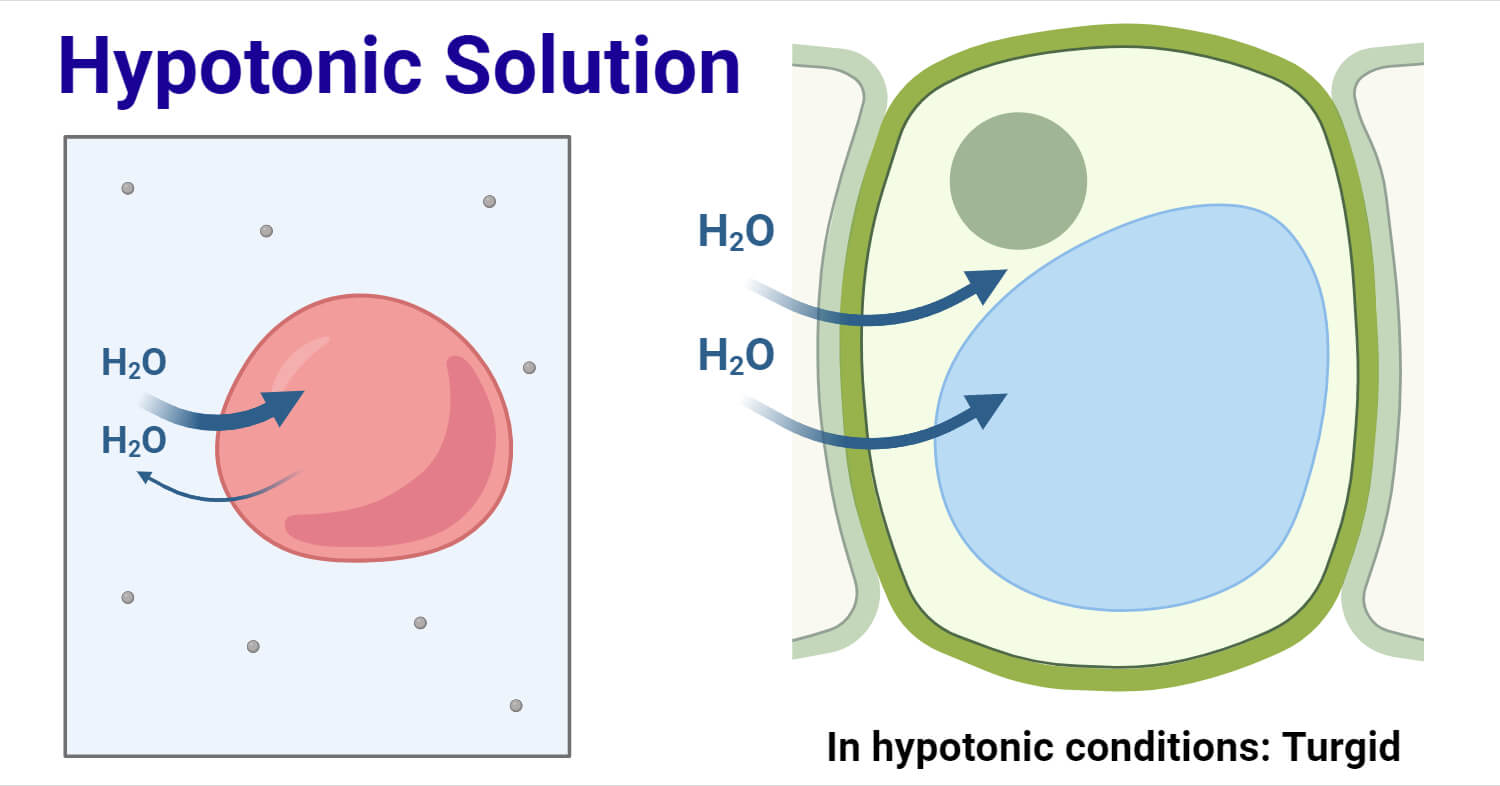
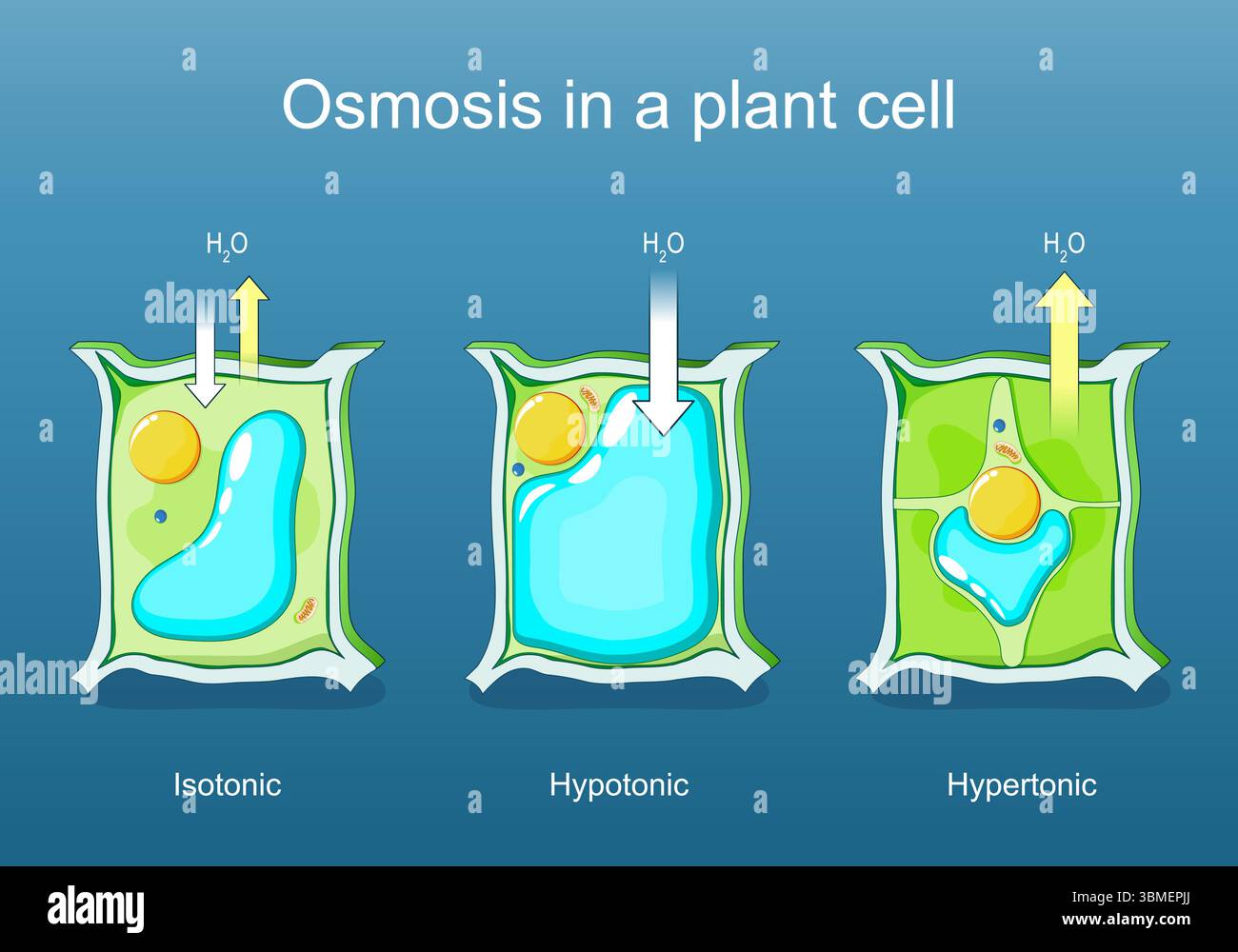
1. Hypotonic Solution (Lower Solute Concentration)
External solution has higher water potential than cell
What happens:
- Water enters the cell by osmosis
- Cell membrane pushes against cell wall
- Cell becomes turgid (swollen and firm)
- Turgor pressure develops
Result:
- Cell maintains shape
- Plant tissues become rigid
- Leaves and stems stand upright
- Healthy appearance
Example:
- Plant cells in pure water
- Well-watered plant cells
- Cells in dilute solutions
2. Isotonic Solution (Equal Solute Concentration)
External solution has same water potential as cell
What happens:
- No net movement of water
- Water enters and leaves at equal rates
- Cell remains in original state
- No turgor pressure
Result:
- Cell is flaccid (limp)
- No net gain or loss of water
- Used as reference point in experiments
Example:
- Cells in solution matching cytoplasm concentration
- Laboratory reference solutions
3. Hypertonic Solution (Higher Solute Concentration)
External solution has lower water potential than cell
What happens:
- Water leaves the cell by osmosis
- Cell membrane pulls away from cell wall
- Cell becomes plasmolyzed
- Cytoplasm shrinks
Result:
- Cell loses shape
- Plant wilts
- Membrane detaches from wall (plasmolysis)
- May recover if placed in water
- Prolonged exposure causes cell death
Example:
- Plant cells in concentrated salt solution
- Plants in drought conditions
- Cells in very salty soil
Turgor Pressure and Its Importance
What is Turgor Pressure?
Turgor pressure is the pressure exerted by the cell contents against the cell wall when water enters the cell by osmosis.
How Turgor Pressure Develops
- Water enters vacuole by osmosis
- Vacuole expands
- Cytoplasm pushes against cell membrane
- Cell membrane pushes against cell wall
- Cell wall resists expansion
- Pressure builds up inside cell
Importance of Turgor Pressure
Structural Support
- Maintains plant shape and rigidity
- Keeps leaves and stems upright
- Non-woody plants rely entirely on turgor
- Wilting occurs when turgor is lost
Cell Growth
- Turgor pressure drives cell expansion
- Cell wall loosens, allowing growth
- Essential for plant development
Opening of Stomata
- Guard cells gain turgor → stomata open
- Guard cells lose turgor → stomata close
- Controls gas exchange and transpiration
Movement Responses
- Some plants use turgor changes for movement
- Venus flytrap closure
- Leaf movements in some species
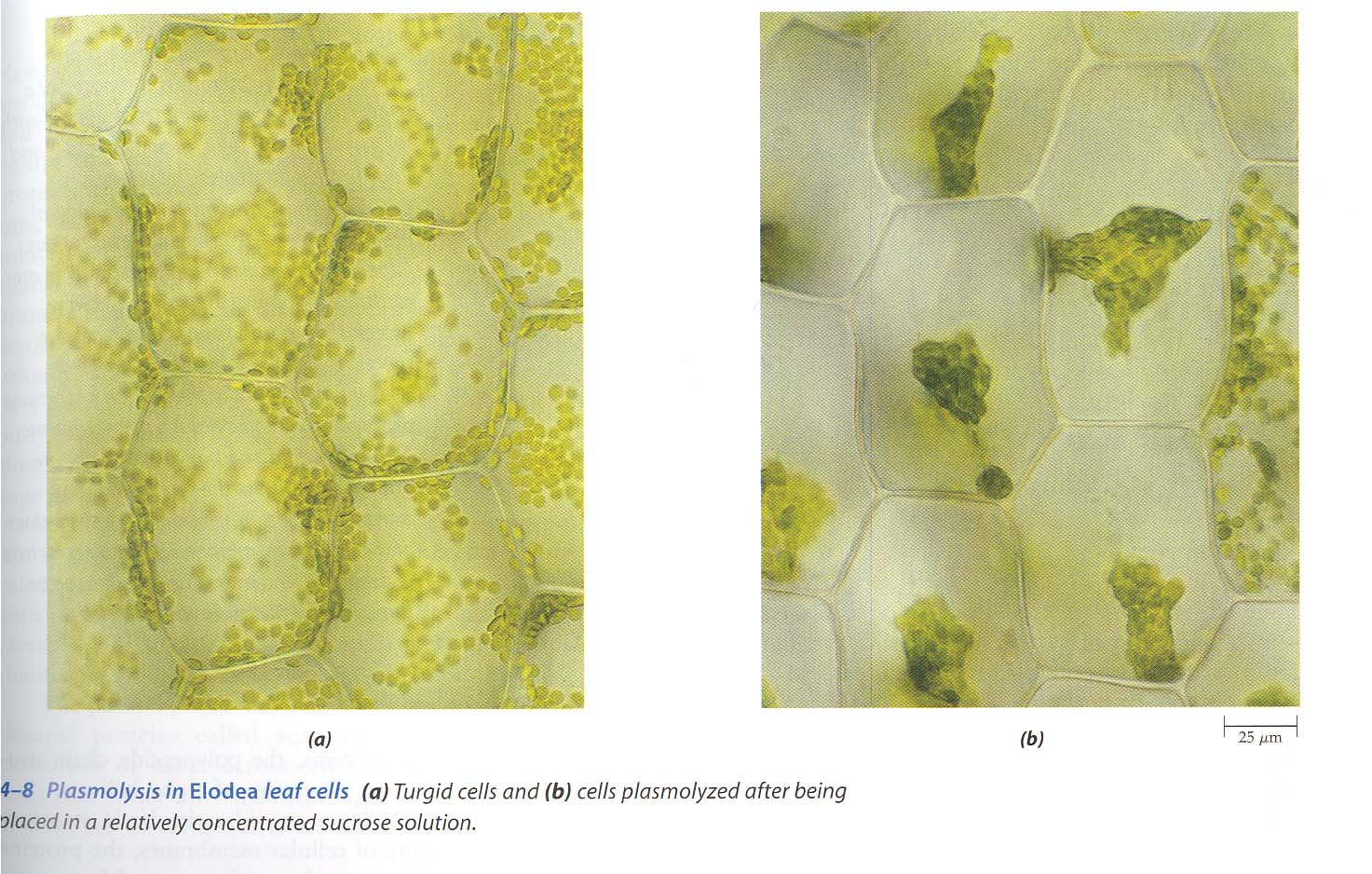
Plasmolysis: When Cells Lose Water
What is Plasmolysis?
Plasmolysis is the process in which the cell membrane pulls away from the cell wall when a plant cell loses water in a hypertonic solution.
Stages of Plasmolysis
Incipient Plasmolysis
- First stage
- Cell membrane just begins to detach
- No visible gap yet
- Cell is flaccid
Evident Plasmolysis
- Clear gap between membrane and wall
- Cytoplasm shrinks
- Visible under microscope
- Cell is limp
Final Plasmolysis
- Maximum shrinkage
- Cytoplasm condensed
- May be irreversible
- Cell may die
Deplasmolysis
- Reverse process
- Cell placed in hypotonic solution
- Water re-enters cell
- Membrane reattaches to wall
- Cell becomes turgid again
- Only possible if cell hasn't been damaged
Osmosis Experiments
Experiment 1: Osmosis in Potato Tissue
Objective: Investigate the effect of solute concentration on osmosis in potato tissue.
Hypothesis: As solute concentration increases, potato tissue will lose mass due to water loss.
Materials:
- Fresh potato
- Cork borer or knife
- Balance (0.01 g precision)
- Ruler
- Beakers or test tubes
- Distilled water
- Salt or sugar
- Measuring cylinder
- Timer
Procedure:
- Preparation:
- Cut potato into equal-sized cylinders (2 cm long, 1 cm diameter)
- Weigh each cylinder and record mass
- Prepare solutions of different concentrations:
- 0% (distilled water)
- 0.2 M salt solution
- 0.4 M salt solution
- 0.6 M salt solution
- 0.8 M salt solution
- 1.0 M salt solution
- Setup:
- Label beakers with concentrations
- Add 50 ml of each solution to appropriate beaker
- Add one potato cylinder to each beaker
- Start timer
- Incubation:
- Leave for 30 minutes (or 1 hour)
- Ensure temperature is constant
- Measurement:
- Remove potato cylinders
- Gently blot dry with paper towel
- Weigh each cylinder
- Record final mass
- Calculation:plain
Percentage change in mass = [(Final - Initial) ÷ Initial] × 100
Expected Results:
| Concentration (M) | Initial Mass (g) | Final Mass (g) | Change (%) | Observation |
|---|---|---|---|---|
| 0.0 | 5.00 | 5.50 | +10% | Turgid, firm |
| 0.2 | 5.00 | 5.20 | +4% | Slightly turgid |
| 0.4 | 5.00 | 4.90 | -2% | Slightly flaccid |
| 0.6 | 5.00 | 4.60 | -8% | Flaccid |
| 0.8 | 5.00 | 4.30 | -14% | Very flaccid |
| 1.0 | 5.00 | 4.00 | -20% | Plasmolyzed |
Graph:
- X-axis: Solute concentration (M)
- Y-axis: Percentage change in mass (%)
- Curve should cross zero at isotonic point
Conclusion:
- Higher concentration = more water loss
- Isotonic point where line crosses zero
- Confirms osmosis theory
Experiment 2: Plasmolysis in Red Onion Cells
Objective: Observe plasmolysis in plant cells using a microscope.
Materials:
- Red onion
- Microscope
- Microscope slides and coverslips
- Dropper
- 1 M salt solution
- Distilled water
- Filter paper
- Forceps
Procedure:
- Prepare slide:
- Peel thin layer from red onion (inner epidermis)
- Place on microscope slide
- Add drop of distilled water
- Add coverslip
- Observe normal cells:
- Focus under low power (10x)
- Switch to high power (40x)
- Draw what you see
- Note: Red color from anthocyanin in vacuole
- Add salt solution:
- Place filter paper at one edge of coverslip
- Add salt solution at opposite edge
- Solution will flow under coverslip
- Wait 2-3 minutes
- Observe plasmolysis:
- Focus on same area
- Draw what you see
- Note: Cell membrane pulls away from wall
- Reverse process (deplasmolysis):
- Add distilled water using same method
- Wait 2-3 minutes
- Observe recovery
Expected Observations:
Normal Cell (in water):
- Cell wall visible as clear boundary
- Cell membrane pressed against wall
- Red cytoplasm fills cell
- Nucleus may be visible
- Cell appears turgid
Plasmolyzed Cell (in salt):
- Cell wall maintains shape
- Cell membrane pulls away from wall
- Cytoplasm shrinks
- Gap between membrane and wall
- Red color concentrated in center
Recovery (back in water):
- Membrane reattaches to wall
- Cytoplasm expands
- Cell returns to normal appearance
Labeled Diagram:
plain
Normal Cell: Plasmolyzed Cell:
┌──────────┐ ┌──────────┐
│ ╭────╮ │ │ ╭──╮ │
│ │████│ │ │ │██│ │
│ │████│ │ │ ╰──╯ │
│ ╰────╯ │ │ │
└──────────┘ └──────────┘
^ Cell wall ^ Cell wall
^ Membrane ^ Membrane (shrunk)
^ Cytoplasm ^ Shrunk cytoplasmExperiment 3: Osmosis Using Visking Tubing
Objective: Demonstrate osmosis using an artificial partially permeable membrane.
Materials:
- Visking tubing (dialysis tubing)
- Beaker
- Funnel
- Clamp stand
- 20% glucose solution
- Distilled water
- Starch solution
- Iodine solution
- Benedict's solution
- Test tubes
Procedure:
- Prepare visking tubing:
- Soak visking tubing in water to soften
- Tie one end securely
- Open other end with funnel
- Fill with solution:
- Add 20% glucose solution + starch solution
- Tie other end securely
- Rinse outside with water
- Record initial appearance
- Set up:
- Fill beaker with distilled water
- Add iodine to water (pale yellow)
- Immerse visking tubing bag
- Leave for 30 minutes
- Observations:
- Color of water (outside)
- Color of contents (inside)
- Test water for glucose (Benedict's test)
Expected Results:
- Water turns blue-black (iodine enters, starch stays in)
- Contents remain colorless (starch too large to exit)
- Water tests positive for glucose (glucose exits)
- Bag may swell slightly (water enters by osmosis)
Conclusions:
- Iodine molecules (small) pass through membrane
- Starch molecules (large) cannot pass
- Glucose (small) passes through
- Water moves by osmosis
Experiment 4: Effect of Temperature on Osmosis
Objective: Investigate how temperature affects the rate of osmosis.
Materials:
- Potato cylinders
- 0.5 M salt solution
- Water baths at different temperatures
- Beakers
- Balance
- Timer
Procedure:
- Prepare identical potato cylinders
- Set up water baths: 5°C, 20°C, 40°C, 60°C
- Place cylinders in 0.5 M salt at each temperature
- Remove after 30 minutes
- Weigh and calculate mass change
Expected Results:
- Higher temperature = faster osmosis
- 40°C likely shows fastest rate
- 60°C may show unusual results (membrane damage)
Explanation:
- Higher temperature increases kinetic energy
- Water molecules move faster
- Rate of osmosis increases
- But excessive heat damages membrane
Factors Affecting Osmosis Rate
1. Concentration Gradient
- Steeper gradient = faster osmosis
- Greater difference in water potential
- Most significant factor
2. Temperature
- Higher temperature = faster osmosis
- Increases kinetic energy
- Until membrane is damaged
3. Surface Area
- Larger surface area = faster osmosis
- More membrane for water to cross
- Thin tissues work faster than thick
4. Pressure
- Pressure against gradient slows osmosis
- Turgor pressure opposes water entry
- Explains why cells don't burst
5. Membrane Permeability
- Damage increases permeability
- Living vs. dead cells differ
- Affects selectivity
Real-World Applications of Osmosis
In Agriculture
Irrigation Management
- Understanding soil water potential
- Preventing waterlogging (hypoxia)
- Optimizing water use efficiency
- Salinity management
Fertilizer Application
- Fertilizer concentration affects water uptake
- Too concentrated causes plasmolysis
- "Fertilizer burn" is osmotic damage
Seed Germination
- Water uptake by osmosis triggers germination
- Seed coat controls water entry
- Essential first step in growth
In Food Preservation
Salting and Sugaring
- High solute concentration draws water out
- Prevents microbial growth
- Traditional preservation methods
- Examples: Pickles, jams, cured meats
Drying
- Removes water from food
- Creates hypertonic environment
- Inhibits bacterial growth
In Medicine
Dialysis
- Artificial kidney uses osmosis principles
- Removes waste from blood
- Partially permeable membrane
IV Solutions
- Must be isotonic with blood
- Prevent cell damage
- Saline (0.9% NaCl) is isotonic
Contact Lens Solution
- Isotonic to prevent eye irritation
- Matches tear composition
In Biology Research
Cell Culture
- Maintain proper osmotic balance
- Isotonic media essential
- Prevents cell death
Protein Purification
- Dialysis removes small molecules
- Concentrates proteins
- Uses osmotic principles
Common Misconceptions About Osmosis
Misconception 1: "Water moves to equalize concentrations"
Correction: Water moves to equalize water potential, not concentrations. Solutes don't move to equalize.
Misconception 2: "Solutes move during osmosis"
Correction: Only water moves during osmosis. Solutes may move by diffusion, but that's different.
Misconception 3: "Osmosis requires energy"
Correction: Osmosis is passive transport. No ATP required. Water moves down water potential gradient.
Misconception 4: "Plant cells burst in pure water"
Correction: Cell wall prevents bursting. Animal cells burst (lyse), plant cells become turgid.
Calculations in Osmosis
Water Potential Equation
plain
Ψ = Ψs + ΨpWhere:
- Ψ = Total water potential
- Ψs = Solute potential (always negative)
- Ψp = Pressure potential (turgor pressure, usually positive)
Example Calculation
Given:
- Solute potential (Ψs) = -500 kPa
- Pressure potential (Ψp) = +300 kPa
Calculation:
plain
Ψ = -500 kPa + 300 kPa = -200 kPaPercentage Change in Mass
plain
% Change = [(Final Mass - Initial Mass) ÷ Initial Mass] × 100Example
- Initial mass = 4.5 g
- Final mass = 4.0 g
plain
% Change = [(4.0 - 4.5) ÷ 4.5] × 100 = (-0.5 ÷ 4.5) × 100 = -11.1%Conclusion
Osmosis is a fundamental biological process essential for plant survival. Understanding how water moves across partially permeable membranes helps explain:
- Why plants wilt in drought
- How roots absorb water from soil
- Why fertilizer concentration matters
- How cells maintain turgor pressure
Through simple experiments with potato tissue, onion cells, and visking tubing, students can observe osmosis in action and develop a deeper appreciation for this vital process. These investigations also demonstrate important scientific skills including experimental design, data collection, and analysis.
Whether you're studying biology, working in agriculture, or simply curious about how plants work, understanding osmosis provides essential insights into plant physiology and water relations.
Osmosis in Plants: Definition and Detailed Explanation
What is Osmosis? Osmosis is the net movement of water molecules from a region of higher water potential (lower solute concentration) to a region of lower water potential (higher solute concentration) across a partially permeable membrane. This is a passive process—no energy (like ATP) is required. It occurs to balance water potential on both sides of the membrane.
In plants, this process is crucial for water uptake, cell rigidity, nutrient transport, and overall plant health.
Key Definitions
- Water Potential (Ψ): A measure of the tendency of water to move. Pure water has Ψ = 0 kPa (highest). Adding solutes makes it negative (lower). Water moves from less negative (higher Ψ) to more negative (lower Ψ).
- Partially Permeable Membrane: Allows water to pass freely but restricts larger solute molecules (e.g., cell/plasma membrane in plants, often with aquaporins aiding water flow).
- Solute: Dissolved substances (e.g., salts, sugars) that lower water potential.
- Solvent: Usually water in biological systems.
Osmosis in Plant Cells












+by+Blacklotus+Landscaping.jpg)


.jpg)


0 Comments