X-RAY CRYSTALLOGRAPHY
Definition:
X-ray crystallography is a technique in which X-rays are directed at crystals, and the atoms/molecules in the crystals diffract the X-rays. From the angles and intensities of these diffracted beams, a 3D electron density map is generated, which is then used to determine the 3D structure of the molecule.

- William Henry Bragg & William Lawrence Bragg
- Developed: 1912
- Nobel Prize in Physics: 1915
Applications:
- Proteins, nucleic acids, carbohydrates, vitamins
- Minerals, salts, metals
- Widely used in structural biology and drug design
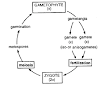
Steps in Protein Structure Determination
Protein Crystallization- Proteins are converted from purified solution into solid crystals.
- Crystals are important because they arrange protein molecules in a regular, repeating pattern, which is necessary for clear X-ray diffraction.
- Process: slowly mix protein with special solutions → carefully control temperature, pH, and salt concentration.
- Time required: hours, days, or even weeks.
Production of Diffraction Pattern
- High-quality crystal is mounted on an X-ray machine.
- X-rays are directed at the crystal → atoms in the crystal diffract the X-rays → a diffraction pattern forms (a series of spots).
Creating Density Map
- The angles and intensities of the spots provide information about the arrangement of atoms in the crystal.
- This information is used to create an electron density map.
Determination of Protein Structure
- Computational programs are used to analyze the data mathematically.
- Diffraction data is converted into the 3D structure of the protein.
Key Points
- Why crystals?
- Proteins need to be arranged in an orderly pattern for clear imaging.
Electron density map:
- Shows the 3D arrangement of atoms in the molecule.
Computational analysis:
- Converts diffraction patterns into 3D structures.
Significance:
- Crucial for understanding protein structure, enzyme function, drug discovery, and molecular biology research.



+by+Blacklotus+Landscaping.jpg)


.jpg)


0 Comments